Dr. Katharina Scherschel
Scientist
Molecular biologist located in Düsseldorf – enthusiastic about neurons in the heart.
Scientific Study Coordinator at the Division of Cardiology, Evangelisches Krankenhaus Düsseldorf. Junior Research Group Leader at the Institute for Neural and Sensory Physiology, Heinrich-Heine University Düsseldorf.
Research focus: To improve the understanding of the cardiac autonomic nervous system in order to help patients with cardiac arrhythmias.
Feel free to look me up and reach out:
Publications
Glia of the heart’s nervous system
Nature Reviews Neuroscience, October 2024
Scherschel K, Wolf H, Ajijola OA, Shivkumar K, Lindner D, Gomez-Sanchez JA, Meyer C
Abstract: The heart adapts to changing physiological demands through bidirectional interactions with the brain. These are mediated via extensive feedback loops of the cardiac autonomic nervous system, a complex network of neurons and glial cells. Although the presence of glia in the heart and its nervous system has been known for decades, only recently has an understanding of their contribution to cardiac physiology and pathophysiology emerged. As new types of cardiac glia are discovered, it becomes evident that they represent heterogeneous cell populations in distinct anatomical locations of the cardiac nervous system, contributing not only to autonomic control of the healthy heart but also to pathological changes in the diseased heart.
Aging impairs the neurovascular interface in the heart
Science, August 2023
Wagner JUG, Tombor LS, Malacarne PF, Kettenhausen LM, Panthel J, Kujundzic H, Manickam N, Schmitz K, Cipca M, Stilz KA, Fischer A, Muhly-Reinholz M, Abplanalp WT, John D, Mohanta SK, Weber C, Habenicht AJR, Buchmann GK, Angendohr S, Amin E, Scherschel K, Klöcker N, Kelm M, Schüttler D, Clauss S, Günther S, Boettger T, Braun T, Bär C, Pham MD, Krishnan J, Hille S, Müller OJ, Bozoglu T, Kupatt C, Nardini E, Osmanagic-Myers S, Meyer C, Zeiher AM, Brandes RP, Luxán G, Dimmeler S
Abstract: Aging is a major risk factor for impaired cardiovascular health. Because the aging myocardium is characterized by microcirculatory dysfunction, and because nerves align with vessels, we assessed the impact of aging on the cardiac neurovascular interface. We report that aging reduces nerve density in the ventricle and dysregulates vascular-derived neuroregulatory genes. Aging down-regulates microRNA 145 (miR-145) and derepresses the neurorepulsive factor semaphorin-3A. miR-145 deletion, which increased Sema3a expression or endothelial Sema3a overexpression, reduced axon density, mimicking the aged-heart phenotype. Removal of senescent cells, which accumulated with chronological age in parallel to the decline in nerve density, rescued age-induced denervation, reversed Sema3a expression, preserved heart rate patterns, and reduced electrical instability. These data suggest that senescence-mediated regulation of nerve density contributes to age-associated cardiac dysfunction.
Pulmonary Vein Isolation by Pulsed-field Ablation Induces Less Neurocardiac Damage Than Cryoballoon Ablation
Circulation: Arrhythmia and Electrophysiology, April 2023
Lemoine MD, Mencke C, Nies M, Obergassel J, Scherschel K, Wieboldt H, Schleberger R, My I, Rottner L, Moser J, Kany S, Wenzel JP, Moser F, Dinshaw L, Münkler P, Reissmann B, Ouyang F, Meyer C, Blankenberg S, Zeller T, Fabritz L, Rillig A, Metzner A, Kirchhof P
Acute Modulation of Left Ventricular Control by Selective Intracardiac Sympathetic Denervation
JACC: Clinical Electrophysiology, March 2023
Kahle AK, Klatt N, Jungen C, Dietenberger A, Kuklik P, Münkler P, Willems S, Nikolaev V, Pauza DH, Scherschel K, Meyer C
Abstract: The sympathetic nervous system plays an integral role in cardiac physiology. Nerve fibers innervating the left ventricle are amenable to transvenous catheter stimulation along the coronary sinus (CS). The aim of the present study was to modulate left ventricular control by selective intracardiac sympathetic denervation. First, the impact of epicardial CS ablation on cardiac electrophysiology was studied in a Langendorff model of decentralized murine hearts (n = 10 each, ablation and control groups). Second, the impact of transvenous, anatomically driven axotomy by catheter-based radiofrequency ablation via the CS was evaluated in healthy sheep (n = 8) before and during stellate ganglion stimulation. Conclusions: Transvenous, anatomically driven axotomy targeting nerve fibers along the CS enables acute modulation of left ventricular control by selective intracardiac sympathetic denervation.
Repolarization indicates electrical instability in ventricular arrhythmia originating from papillary muscle
EP Europace, February 2023
Münkler P, Klatt N, Scherschel K, Kuklik P, Jungen C, Cavus E, Eickholt C, Christoph J, Lemoine MD, Christ T, Willems S, Riedel R, Kirchhof P, Meyer C
Abstract: Cardiac arrhythmia originating from the papillary muscle (PM) can trigger ventricular fibrillation (VF) and cause sudden cardiac death even in the absence of structural heart disease. Most premature ventricular contractions, however, are benign and hitherto difficult to distinguish from a potentially fatal arrhythmia. Altered repolarization characteristics are associated with electrical instability, but electrophysiological changes which precede degeneration into VF are still not fully understood. Ventricular arrhythmia (VA) was induced by aconitine injection into PMs of healthy sheep. To investigate mechanisms of degeneration of stable VA into VF in structurally healthy hearts, endocardial high-density and epicardial mapping was performed during sinus rhythm (SR) and VA. The electrical restitution curve, modelling the relation of diastolic interval and activation recovery interval (a surrogate parameter for action potential duration), is steeper in VA than in non-arrhythmia (ventricular pacing and SR). Steeper restitution curves reflect electrical instability and propensity to degenerate into VF. Importantly, we find the parameter repolarization time in relation to cycle length (RT/CL) to differentiate self-limiting from degenerating arrhythmia with high specificity and sensitivity. RT/CL may serve as a simple index to aid differentiation between self-limiting and electrically instable arrhythmia with the propensity to degenerate to VF. RT/CL is independent of cycle length and could easily be measured to identify electrical instability in patients.
In-hospital mortality and major complications related to radiofrequency catheter ablations of over 10 000 supraventricular arrhythmias from 2005 to 2020: individualized case analysis of multicentric administrative data
EP Europace, February 2023
Doldi F, Geßler N, Anwar O, Kahle AK, Scherschel K, Rath B, Köbe J, Lange PS, Frommeyer G, Metzner A, Meyer C, Willems S, Kuck KH, Eckardt L
Abstract: The incidence of in-hospital post-interventional complications and mortality after ablation of supraventricular tachycardia (SVT) vary among the type of procedure and most likely the experience of the centre. As ablation therapy of SVT is progressively being established as first-line therapy, further assessment of post-procedural complication rates is crucial for health care quality. We aimed at determining the incidence of in-hospital mortality and bleeding complications from SVT ablations in German high-volume electrophysiological centres between 2005 and 2020. All cases were registered by the German Diagnosis Related Groups-and the German Operation and Procedure Classification (OPS) system. A uniform search for SVT ablations from 2005 to 2020 with the same OPS codes defining the type of ablation/arrhythmia as well as the presence of a vascular complication, cardiac tamponade, and/or in-hospital death was performed. An overall of 47 610 ablations with 10 037 SVT ablations were registered from 2005 to 2020 among three high-volume centres. An overall complication rate of 0.5% (n = 38) was found [median age, 64; ±15 years; female n = 26 (68%)]. All-cause mortality was 0.02% (n = 2) and both patients had major prior co-morbidities precipitating a lethal outcome irrespective of the ablation procedure. Vascular complications occurred in 10 patients (0.1%), and cardiac tamponade was detected in 26 cases (0.3%). The present case-based analysis shows an overall low incidence of in-hospital complications after SVT ablation highlighting the overall very good safety profile of SVT ablations in high-volume centres. Further prospective analysis is still warranted to guarantee continuous quality control and optimal patient care.
Combined contact force and local impedance dynamics during repeat atrial fibrillation catheter ablation
Frontiers in Physiology, October 2022
Alken FA, Scherschel K, Kahle AK, Masjedi M, Meyer C
Abstract: Optimal lesion formation during catheter-based radiofrequency current (RFC) ablation depends on electro-mechanical tip-tissue coupling measurable via contact force (CF) and local impedance (LI) monitoring. We aimed to investigate CF and LI dynamics in patients with previous atrial fibrillation (AF) ablation who frequently present with heterogenous arrhythmia substrate. Data from consecutive patients presenting for repeat AF or atrial tachycardia ablation using a novel open-irrigated single-tip ablation catheter were studied. RFC applications were investigated regarding CF, LI and the maximum LI drop (∆LI) for evaluation of ablation efficacy. ∆LI > 20 Ω was defined as a successful RFC application. Conclusion: Combination of CF and LI may aid monitoring real-time catheter-tissue electro-mechanical coupling and lesion formation within heterogenous atrial arrhythmia substrate in patients with repeat AF or atrial tachycardia ablation.
Respiratory and heart rate dynamics during peripheral chemoreceptor deactivation compared to targeted sympathetic and sympathetic/parasympathetic (co-)activation
Autonomic Neuroscience, September 2022
Apelt-Glitz K, Alken FA, Jungen C, Scherschel K, Klöcker N, Meyer C
Abstract: The importance of peripheral chemoreceptors for cardiorespiratory neural control is known for decades. Pure oxygen inhalation deactivates chemoreceptors and increases parasympathetic outflow. However, the relationship between autonomic nervous system (ANS) activation and resulting respiratory as well as heart rate (HR) dynamics is still not fully understood. In young adults the impact of (1) 100 % pure oxygen inhalation (hyperoxic cardiac chemoreflex sensitivity (CHRS) testing), (2) the cold face test (CFT) and (3) the cold pressor test (CPT) on heart rate variability (HRV), hemodynamics and respiratory rate was investigated in randomized order. Baseline ANS outflow was determined assessing respiratory sinus arrhythmia via deep breathing, baroreflex sensitivity and HRV. Conclusion: Changes in HR characteristics during deactivation of peripheral chemoreceptors but not during the CFT and CPT are related with a decrease in respiratory rate. This highlights the need of respiratory rate assessment when evaluating adaptations of cardiorespiratory chemoreceptor control.
Bipolar ablation of therapy-refractory ventricular arrhythmias: application of a dedicated approach
EP Europace, June 2022
Kany S, Alken FA, Schleberger R, Baran J, Luik A, Haas A, Ene E, Deneke T, Dinshaw L, Rillig A, Metzner A, Reissmann B, Makimoto H, Reents T, Popa MA, Deisenhofer I, Piotrowski R, Kulakowski P, Kirchhof P, Scherschel K, Meyer C
Abstract: Bipolar radiofrequency ablation (B-RFA) has been reported as a bail-out strategy for the treatment of therapy refractory ventricular arrhythmias (VA). Currently, existing setups have not been standardized for B-RFA, while the impact of conventional B-RFA approaches on lesion formation remains unclear. Using a dedicated device for B-RFA for therapy-refractory VA appears feasible and safe. While some patients need repeat ablation, success rates were encouraging. Sequential unipolar and B-RFA may be favourable for lesion formation.
Relationship Between Early and Late Recurrences After Catheter Ablation for Atrial Tachycardia in Patients With a History of Atrial Fibrillation
Circulation: Arrythmia and Electrophysiology, May 2022
Kahle AK, Jungen C, Scherschel K, Alken FA, Meyer C
Management of ventricular tachycardia in patients with ischaemic cardiomyopathy: contemporary armamentarium
EP Europace, April 2022
Scherschel K, Bräuninger H, Mölders A, Erlenhardt N, Amin E, Jungen C, Pape U, Lindner D, Chetkovich DM, Klöcker N, Meyer C
Abstract: Worldwide, ∼4 million people die from sudden cardiac death every year caused in more than half of the cases by ischaemic cardiomyopathy (ICM). Prevention of sudden cardiac death after myocardial infarction by implantation of a cardioverter-defibrillator (ICD) is the most common, even though not curative, therapy to date. Optimized ICD programming should be strived for in order to decrease the incidence of ICD interventions. Catheter ablation reduces the recurrence of ventricular tachycardias (VTs) and is an important adjunct to sole ICD-based treatment or pharmacological antiarrhythmic therapy in patients with ICM, as conclusively demonstrated by seven randomized controlled trials (RCTs) in the last two decades. However, none of the conducted trials was powered to reveal a survival benefit for ablated patients as compared to controls. Whereas thorough consideration of an early approach is necessary following two recent RCTs (PAUSE-SCD, BERLIN VT), catheter ablation is particularly recommended in patients with recurrent VT after ICD therapy. In this context, novel, pathophysiologically driven ablation strategies referring to deep morphological and functional substrate phenotyping based on high-resolution mapping and three-dimensional visualization of scars appear promising. Emerging concepts like sympathetic cardiac denervation as well as radioablation might expand the therapeutical armamentarium especially in patients with therapy-refractory VT. Randomized controlled trials are warranted and on the way to investigate how these translate into improved patient outcome. This review summarizes therapeutic strategies currently available for the prevention of VT recurrences, the optimal timing of applicability, and highlights future perspectives after a PAUSE in BERLIN.
Cytokine-Mediated Alterations of Human Cardiac Fibroblast's Secretome
International Journal of Molecular Sciences, November 2021
Bräuninger H, Thottakara T, Schön J, Voss S, Dhople V, Warnke S, Scherschel K, Schrage B, Kirchhof P, Blankenberg S, Völker U, Westermann D, Hammer E, Lindner D
Abstract: Fibroblasts contribute to approximately 20% of the non-cardiomyocytic cells in the heart. They play important roles in the myocardial adaption to stretch, inflammation, and other pathophysiological conditions. Fibroblasts are a major source of extracellular matrix (ECM) proteins whose production is regulated by cytokines, such as TNF-α or TGF-β. The resulting myocardial fibrosis is a hallmark of pathological remodeling in dilated cardiomyopathy (DCM). Therefore, in the present study, the secretome and corresponding transcriptome of human cardiac fibroblasts from patients with DCM was investigated under normal conditions and after TNF-α or TGF-β stimulation. Secreted proteins were quantified via mass spectrometry and expression of genes coding for secreted proteins was analyzed via Affymetrix Transcriptome Profiling. Thus, we provide comprehensive proteome and transcriptome data on the human cardiac fibroblast’s secretome. In the secretome of quiescent fibroblasts, 58% of the protein amount belonged to the ECM fraction. Interestingly, cytokines were responsible for 5% of the total protein amount in the secretome and up to 10% in the corresponding transcriptome. Furthermore, cytokine gene expression and secretion were upregulated upon TNF-α stimulation, while collagen secretion levels were elevated after TGF-β treatment. These results suggest that myocardial fibroblasts contribute to pro-fibrotic and to inflammatory processes in response to extracellular stimuli.
Cardiac SARS-CoV-2 infection is associated with pro-inflammatory transcriptomic alterations within the heart
Cardiovascular Research, October 2021
Bräuninger H, Stoffers B, Fitzek ADE, Meißner K, Aleshcheva G, Schweizer M, Weimann J, Rotter B, Warnke S, Edler C, Braun F, Roedl K, Scherschel K, Escher F, Kluge S, Huber TB, Ondruschka B, Schultheiss HP, Kirchhof P, Blankenberg S, Püschel K, Westermann D, Lindner D
Abstract: Cardiac involvement in COVID-19 is associated with adverse outcome. However, it is unclear whether cell-specific consequences are associated with cardiac SARS-CoV-2 infection. Therefore, we investigated heart tissue utilizing in situ hybridization, immunohistochemistry, and RNA-sequencing in consecutive autopsy cases to quantify virus load and characterize cardiac involvement in COVID-19. In this study, 95 SARS-CoV-2-positive autopsy cases were included. A relevant SARS-CoV-2 virus load in the cardiac tissue was detected in 41/95 deceased (43%). Massive analysis of cDNA ends (MACE)-RNA-sequencing was performed to identify molecular pathomechanisms caused by the infection of the heart. A signature matrix was generated based on the single-cell dataset ‘Heart Cell Atlas’ and used for digital cytometry on the MACE-RNA-sequencing data. Thus, immune cell fractions were estimated and revealed no difference in immune cell numbers in cases with and without cardiac infection. This result was confirmed by quantitative immunohistological diagnosis. MACE-RNA-sequencing revealed 19 differentially expressed genes (DEGs) with a q-value <0.05 (e.g. up: IFI44L, IFT3, TRIM25; down: NPPB, MB, MYPN). The upregulated DEGs were linked to interferon pathways and originate predominantly from endothelial cells. In contrast, the downregulated DEGs originate predominately from cardiomyocytes. Immunofluorescent staining showed viral protein in cells positive for the endothelial marker ICAM1 but rarely in cardiomyocytes. The Gene Ontology (GO) term analysis revealed that downregulated GO terms were linked to cardiomyocyte structure, whereas upregulated GO terms were linked to anti-virus immune response. This study reveals that cardiac infection induced transcriptomic alterations mainly linked to immune response and destruction of cardiomyocytes. While endothelial cells are primarily targeted by the virus, we suggest cardiomyocyte destruction by paracrine effects. Increased pro-inflammatory gene expression was detected in SARS-CoV-2-infected cardiac tissue but no increased SARS-CoV-2 associated immune cell infiltration was observed.
A novel algorithm for 3-D visualization of electrogram duration for substrate-mapping in patients with ischemic heart disease and ventricular tachycardia
PLOS One, July 2021
Masjedi M, Jungen C, Kuklik P, Alken FA, Kahle AK, Klatt N, Scherschel K, Lorenz J, Meyer C
Abstract: Myocardial slow conduction is a cornerstone of ventricular tachycardia (VT). Prolonged electrogram (EGM) duration is a useful surrogate parameter and manual annotation of EGM characteristics are widely used during catheter-based ablation of the arrhythmogenic substrate. However, this remains time-consuming and prone to inter-operator variability. We aimed to develop an algorithm for 3-D visualization of EGM duration relative to the 17-segment American Heart Association model. To calculate and visualize EGM duration, in sinus rhythm acquired high-density maps of patients with ischemic cardiomyopathy undergoing substrate-based VT ablation using a 64-mini polar basket-catheter with low noise of 0.01 mV were analyzed. Using a custom developed algorithm based on standard deviation and threshold, the relationship between EGM duration, endocardial voltage and ablation areas was studied by creating 17-segment 3-D models and 2-D polar plots. Conclusion: The novel algorithm allows rapid visualization of prolonged EGM durations. This may facilitate more objective characterization of arrhythmogenic substrate in patients with ischemic cardiomyopathy.
Characterization of the HCN Interaction Partner TRIP8b/PEX5R in the Intracardiac Nervous System of TRIP8b-Deficient and Wild-Type Mice
International Journal of Molecular Sciences, April 2021
Scherschel K, Bräuninger H, Mölders A, Erlenhardt N, Amin E, Jungen C, Pape U, Lindner D, Chetkovich DM, Klöcker N, Meyer C
Abstract: The tetratricopeptide repeat-containing Rab8b-interacting protein (TRIP8b/PEX5R) is an interaction partner and auxiliary subunit of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which are key for rhythm generation in the brain and in the heart. Since TRIP8b is expressed in central neurons but not in cardiomyocytes, the TRIP8b-HCN interaction has been studied intensely in the brain, but is deemed irrelevant in the cardiac conduction system. Still, to date, TRIP8b has not been studied in the intrinsic cardiac nervous system (ICNS), a neuronal network located within epicardial fat pads. In vitro electrophysiological studies revealed that TRIP8b-deficient mouse hearts exhibit increased atrial refractory and atrioventricular nodal refractory periods, compared to hearts of wild-type littermates. Meanwhile, heart rate, sino-nodal recovery time, and ventricular refractory period did not differ between genotypes. Trip8b mRNA was detected in the ICNS by quantitative polymerase chain reaction. RNAscope in situ hybridization confirmed Trip8b localization in neuronal somata and nerve fibers. Additionally, we found a very low amount of mRNAs in the sinus node and atrioventricular node, most likely attributable to the delicate fibers innervating the conduction system. In contrast, TRIP8b protein was not detectable. Our data suggest that TRIP8b in the ICNS may play a role in the modulation of atrial electrophysiology beyond HCN-mediated sino-nodal control of the heart.
Location, Dissection, and Analysis of the Murine Stellate Ganglion
JoVE Journal, December 2020
Scherschel K, Bräuninger H, Glufke K, Jungen C, Klöcker N, Meyer C
Abstract: The autonomic nervous system is a substantial driver of cardiac electrophysiology. Especially the role of its sympathetic branch is an ongoing matter of investigation in the pathophysiology of ventricular arrhythmias (VA). Neurons in the stellate ganglia (SG) – bilateral star-shaped structures of the sympathetic chain – are an important component of the sympathetic infrastructure. The SG are a recognized target for treatment via cardiac sympathetic denervation in patients with therapy-refractory VA. While neuronal remodeling and glial activation in the SG have been described in patients with VA, the underlying cellular and molecular processes that potentially precede the onset of arrhythmia are only insufficiently understood and should be elucidated to improve autonomic modulation. Mouse models allow us to study sympathetic neuronal remodeling, but identification of the murine SG is challenging for the inexperienced investigator. Thus, in-depth cellular and molecular biological studies of the murine SG are lacking for many common cardiac diseases. Here, we describe a basic repertoire for dissecting and studying the SG in adult mice for analyses at RNA level (RNA isolation for gene expression analyses, in situ hybridization), protein level (immunofluorescent whole mount staining), and cellular level (basic morphology, cell size measurement). We present potential solutions to overcome challenges in the preparation technique, and how to improve staining via quenching of autofluorescence. This allows for the visualization of neurons as well as glial cells via established markers in order to determine cell composition and remodeling processes. The methods presented here allow characterizing the SG to gain further information on autonomic dysfunction in mice prone to VA and can be complemented by additional techniques investigating neuronal and glial components of the autonomic nervous system in the heart.
Impact of the ablation technique on release of the neuronal injury marker S100B during pulmonary vein isolation
Europace, August 2020
Scherschel K, Hedenus K, Jungen C, Münkler P, Willems S, Anwar O, Klatt N, Eickholt C, Meyer C
Abstract: S100B, a well-known damage-associated molecular pattern protein is released acutely by central and peripheral nerves and upon concomitant denervation in pulmonary vein isolation (PVI). We aimed to investigate whether the ablation technique used for PVI impacts S100B release in patients with paroxysmal atrial fibrillation (AF).
Outcome after tailored catheter ablation of atrial tachycardia using ultra-high-density mapping
Journal of Cardiovascular Electrophysiology, August 2020
Jungen C, Akbulak R, Kahle AK, Eickholt C, Schaeffer B, Scherschel K, Dinshaw L, Muenkler P, Schleberger R, Nies M, Gunawardene MA, Klatt N, Hartmann J, Merbold L, Jularic M, Willems S, Meyer C
Abstract: Tailored catheter ablation of atrial tachycardias (ATs) is increasingly recommended as a potentially easy treatment strategy in the era of high-density mapping (HDM). As follow-up data are sparse, we here report outcomes after HDM-guided ablation of ATs in patients with prior catheter ablation or cardiac surgery.
Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases
JAMA Cardiology, July 2020
Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, Blankenberg S, Püschel K, Westermann D
Abstract: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can be documented in various tissues, but the frequency of cardiac involvement as well as possible consequences are unknown. Cardiac tissue from 39 consecutive autopsy cases were included. The median (interquartile range) age of patients was 85 (78-89) years, and 23 (59.0%) were women. SARS-CoV-2 could be documented in 24 of 39 patients (61.5%). Viral load above 1000 copies per μg RNA could be documented in 16 of 39 patients (41.0%). A cytokine response panel consisting of 6 proinflammatory genes was increased in those 16 patients compared with 15 patients without any SARS-CoV-2 in the heart. Comparison of 15 patients without cardiac infection with 16 patients with more than 1000 copies revealed no inflammatory cell infiltrates or differences in leukocyte numbers per high power field. In this analysis of autopsy cases, viral presence within the myocardium could be documented. While a response to this infection could be reported in cases with higher virus load vs no virus infection, this was not associated with an influx of inflammatory cells. Future investigations should focus on evaluating the long-term consequences of this cardiac involvement.
Contemporary analysis of phrenic nerve injuries following cryoballoon-based pulmonary vein isolation: A single-centre experience with the systematic use of compound motor action potential monitoring
PLOS One, June 2020
Anwar O, Gunawardene MA, Dickow J, Scherschel K, Jungen C, Münkler P, Eickholt C, Willems S, Gessler N, Meyer C
Abstract: Phrenic nerve injury (PNI) remains one of the most frequent complications during cryoballoon-based pulmonary vein isolation (CB-PVI). Since its introduction in 2013, the use of compound motor action potential (CMAP) for the prevention of PNI during CB-PVI is increasing; however, systematic outcome data are sparse. The CMAP technique was applied in conjunction with abdominal palpation during pacing manoeuvres (10 mV, 2 ms) from the superior vena cava for 388 consecutive patients undergoing CB-PVI between January 2015 and May 2017 at our tertiary arrhythmia centre. Cryoablation was immediately terminated when CMAP amplitude was reduced by 30%. Reductions in CMAP amplitude were observed in 16 (4%) of 388 patients during isolation of the right veins. Of these, 11 (69%) patients did not manifest a reduction in diaphragmatic excursions. The drop in CMAP amplitude was observed in 10 (63%) patients during ablation of the right superior pulmonary veins (PVs) and in 7 (44%) patients during ablation of the right inferior PVs. Postprocedural persistent PNI was observed in three of four patients for a duration of 6 months, with one of these patients remaining symptomatic at the 24-month follow-up. One of the four patients was lost to long-term follow-up. All PNIs occurred during right-sided CB-PVI and were preceded by a reduction in CMAP amplitude. Thus, the standardized use of CMAP surveillance during CB-PVI is easily applicable, reliable and compared with other studies, results in a lower number of PNIs.
Rationale and Design of the Hamburg City Health Study
European Journal of Epidemiology, November 2019
Jagodzinski A, Johansen C, Koch-Gromus U, Aarabi G, Adam G, Anders S, Augustin M, der Kellen RB, Beikler T, Behrendt CA, Betz CS, Bokemeyer C, Borof K, Briken P, Busch CJ, Büchel C, Brassen S, Debus ES, Eggers L, Fiehler J, Gallinat J, Gellißen S, Gerloff C, Girdauskas E, Gosau M, Graefen M, Härter M, Harth V, Heidemann C, Heydecke G, Huber TB, Hussein Y, Kampf MO, von dem Knesebeck O, Konnopka A, König HH, Kromer R, Kubisch C, Kühn S, Loges S, Löwe B, Lund G, Meyer C, Nagel L, Nienhaus A, Pantel K, Petersen E, Püschel K, Reichenspurner H, Sauter G, Scherer M, Scherschel K, Schiffner U, Schnabel RB, Schulz H, Smeets R, Sokalskis V, Spitzer MS, Terschüren C, Thederan I, Thoma T, Thomalla G, Waschki B, Wegscheider K, Wenzel JP, Wiese S, Zyriax BC, Zeller T, Blankenberg S
Abstract: The Hamburg City Health Study (HCHS) is a large, prospective, long-term, population-based cohort study and a unique research platform and network to obtain substantial knowledge about several important risk and prognostic factors in major chronic diseases. A random sample of 45,000 participants between 45 and 74 years of age from the general population of Hamburg, Germany, are taking part in an extensive baseline assessment at one dedicated study center. Participants undergo 13 validated and 5 novel examinations primarily targeting major organ system function and structures including extensive imaging examinations. The protocol includes validate self-reports via questionnaires regarding lifestyle and environmental conditions, dietary habits, physical condition and activity, sexual dysfunction, professional life, psychosocial context and burden, quality of life, digital media use, occupational, medical and family history as well as healthcare utilization. The assessment is completed by genomic and proteomic characterization. Beyond the identification of classical risk factors for major chronic diseases and survivorship, the core intention is to gather valid prevalence and incidence, and to develop complex models predicting health outcomes based on a multitude of examination data, imaging, biomarker, psychosocial and behavioral assessments. Participants at risk for coronary artery disease, atrial fibrillation, heart failure, stroke and dementia are invited for a visit to conduct an additional MRI examination of either heart or brain. Endpoint assessment of the overall sample will be completed through repeated follow-up examinations and surveys as well as related individual routine data from involved health and pension insurances. The study is targeting the complex relationship between biologic and psychosocial risk and resilience factors, chronic disease, health care use, survivorship and health as well as favorable and bad prognosis within a unique, large-scale long-term assessment with the perspective of further examinations after 6 years in a representative European metropolitan population.
Advanced mapping strategies for ablation therapy in adults with congenital heart disease
Cardiovascular Diagnosis and Therapy, October 2019
Alken FA, Klatt N, Muenkler P, Scherschel K, Jungen C, Akbulak RO, Kahle AK, Gunawardene M, Jularic M, Dinshaw L, Hartmann J, Eickholt C, Willems S, Stute F, Mueller G, Blankenberg S, Rickers C, Sinning C, Zengin-Sahm E, Meyer C
Abstract: Ultra-high density mapping (HDM) is a promising tool in the treatment of patients with complex arrhythmias. In adults with congenital heart disease (CHD), rhythm disorders are among the most common complications but catheter ablation can be challenging due to heterogenous anatomy and complex arrhythmogenic substrates. Here, we describe our initial experience using HDM in conjunction with novel automated annotation algorithms in patients with moderate to great CHD complexity. Our findings suggest that HDM in conjunction with novel automated annotation algorithms provides detailed insights into arrhythmia mechanisms and might facilitate tailored catheter ablation in patients with moderate to great CHD complexity.
Increased arrhythmia susceptibility in type 2 diabetic mice related to dysregulation of ventricular sympathetic innervation
American Journal of Physiology - Heart and Circulatory Physiology, October 2019
Jungen C, Scherschel K, Flenner F, Jee H, Rajendran PS, De Jong KA, Nikolaev VO, Meyer C, Ardell JL, Tompkins JD
Abstract: Patients with type 2 diabetes mellitus (T2DM) have a greater risk of developing life-threatening cardiac arrhythmias. Since the underlying mechanisms and potential influence of diabetic autonomic neuropathy are not well understood, we aimed to assess the relevance of a dysregulation in cardiac autonomic tone. Methods and results: Ventricular arrhythmia susceptibility was increased in Langendorff-perfused hearts isolated from mice with T2DM (db/db). Membrane properties and synaptic transmission were similar at cardiac postganglionic parasympathetic neurons from diabetic and control mice; however, a greater asynchronous neurotransmitter release was present at sympathetic postganglionic neurons from the stellate ganglia of db/db mice. Western blot analysis showed a reduction of tyrosine hydroxylase (TH) from the ventricles of db/db mice, which was confirmed with confocal imaging as a heterogeneous loss of TH-immunoreactivity from the left ventricular wall but not the apex. In-vivo stimulation of cardiac parasympathetic (vagus) or cardiac sympathetic (stellate ganglion) nerves induced similar changes in heart rate in control and db/db mice and the kinetics of pacing-induced Ca2+ transients, recorded from isolated cardiomyocytes, were similar in control and db/db cells. Antagonism of cardiac muscarinic receptors did not affect the frequency or severity of arrhythmias in db/db mice, but sympathetic blockade with propranolol completely inhibited arrhythmogenicity. Collectively, these findings suggest that the increased ventricular arrhythmia susceptibility of type 2 diabetic mouse hearts is due to dysregulation of the sympathetic ventricular control.
EAT: What role does the fat around the heart play?
International Journal of Cardiology, August 2019
Katharina Scherschel, Nils Gosau
Abstract: Obesity is on the rise, and with it, fatty heart tissue as well. Epicardial adipose tissue (EAT) and its relationship to cardiovascular disease have been attracting more attention over the last decade. EAT is a depot of fat surrounding the heart within the pericardial sack; located predominantly around coronary arteries and the atrioventricular groove as well as at distinct positions surrounding the atria. It is thus divided into peri-ventricular and peri-atrial EAT. The latter, or more specifically, left atrial adipose tissue (LAAT) is the focus of Lopez-Canoa and colleagues in the current issue of the International Journal of Cardiology.
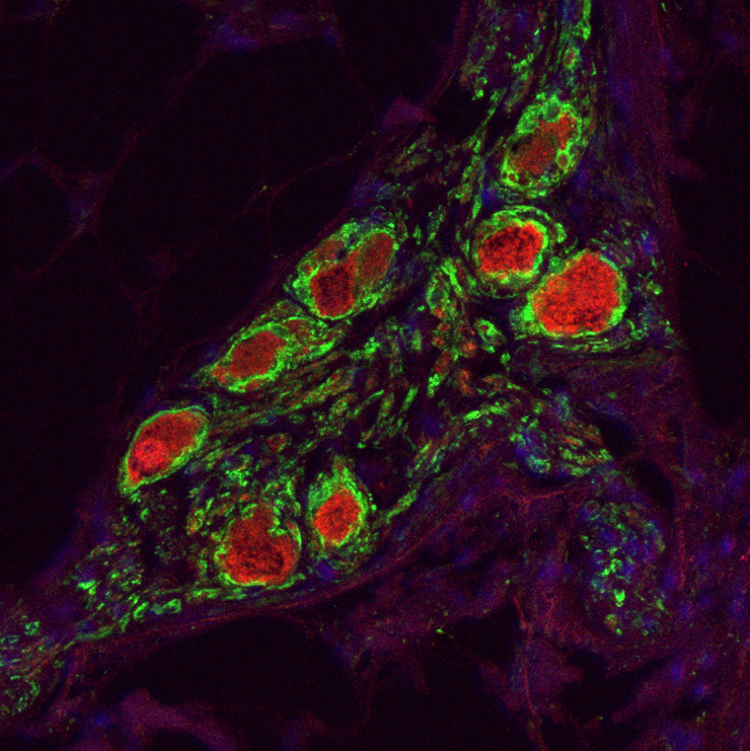
Cardiac glial cells release neurotrophic S100B upon catheter-based treatment of atrial fibrillation
Science Translational Medicine, May 2019
Scherschel K*, Hedenus K*, Jungen C, Lemoine MD, Rübsamen N, Veldkamp MW, Klatt N, Lindner D, Westermann D, Casini S, Kuklik P, Eickholt C, Klöcker N, Shivkumar K, Christ T, Zeller T, Willems S, Meyer C
Abstract: Atrial fibrillation (AF), the most common sustained heart rhythm disorder worldwide, is linked to dysfunction of the intrinsic cardiac autonomic nervous system (ICNS). The role of ICNS damage occurring during catheter-based treatment of AF, which is the therapy of choice for many patients, remains controversial. We show here that the neuronal injury marker S100B is expressed in cardiac glia throughout the ICNS and is released specifically upon catheter ablation of AF. Patients with higher S100B release were more likely to be AF free during follow-up. Subsequent in vitro studies revealed that murine intracardiac neurons react to S100B with diminished action potential firing and increased neurite growth. This suggests that release of S100B from cardiac glia upon catheter-based treatment of AF is a hallmark of acute neural damage that contributes to nerve sprouting and can be used to assess ICNS damage.
Respiratory sinus arrhythmia is reduced after pulmonary vein isolation in patients with paroxysmal atrial fibrillation
Archives of Medical Science, March 2019
Christiane Jungen, Fares-Alexander Alken, Christian Eickholt, Katharina Scherschel, Pawel Kuklik, Niklas Klatt, Jana Schwarzl, Julia Moser, Mario Jularic, Ruken Oezge Akbulak, Benjamin Schaeffer, Stephan Willems, Christian Meyer
Abstract: Respiratory sinus arrhythmia (RSA) describes heart rate (HR) changes in synchrony with respiration. It is relevant for exercise capacity and mechanistically linked with the cardiac autonomic nervous system. After pulmonary vein isolation (PVI), the current therapy of choice for patients with paroxysmal atrial fibrillation (AF), the cardiac vagal tone is often diminished. We hypothesized that RSA is modulated by PVI in patients with paroxysmal AF.
Dissecting hiPSC-CM pacemaker function in a cardiac organoid model
Biomaterials, March 2019
Schulze ML, Lemoine MD, Fischer AW, Scherschel K, David R, Riecken K, Hansen A, Eschenhagen T, Ulmer BM
Abstract: Biological pacemakers could be a promising alternative to electronic pacemakers and human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CM) may represent a suitable source for implantable cells. To further unravel this potential a thorough understanding of pacemaker function with regard to coupling processes both in the physiological and in the graft-host context is required. Here we developed a 2-component cardiac organoid model with a hiPSC-CM embryoid body (EB) as trigger casted into a rat engineered heart tissue (EHT) as arrhythmic beating substrate. Contractility recordings revealed that the EB controlled the beating activity of the EHT, leading to a regular hiPSC-CM-like beating pattern instead of the irregular beating typically seen in rat EHT. Connectivity was observed with action potential (AP) measurements and calcium transients transmitting from the EB directly into the rat EHT. Immunohistochemistry and genetically labeled hiPSC-CMs demonstrated that EB-derived and rat cells intermingled and formed a transitional zone. Connexin 43 expression followed the same pattern as histological and computer models have indicated for the human sinoatrial node. In conclusion, hiPSC-CM EBs function as a biological pacemaker in a 2-component cardiac organoid model, which provides the possibility to study electrophysiological and structural coupling mechanisms underlying propagation of pacemaker activity.
Macrophage Migration Inhibitory Factor (MIF) Expression Increases during Myocardial Infarction and Supports Pro-Inflammatory Signaling in Cardiac Fibroblasts
Biomolecules, January 2019
Voss S, Krüger S, Scherschel K, Warnke S, Schwarzl M, Schrage B, Girdauskas E, Meyer C, Blankenberg S, Westermann D, Lindner D
Abstract: Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine known to play a major role in inflammatory diseases such as myocardial infarction (MI), where its expression increases. Cardio protective functions of MIF during ischemia have been reported. Recently, the structurally related MIF-2 was identified and similar effects are assumed. We wanted to further investigate the role of MIF and MIF-2 on inflammatory processes during MI. Therefore, we subjected mice to experimentally induced MI by coronary occlusion for one and five days. During the acute phase of MI, the gene expression of Mif was upregulated in the infarct zone, whereas Mif-2 was downregulated, suggesting a minor role of MIF-2. Simulating ischemic conditions or mechanical stress in vitro, we demonstrated that Mif expression was induced in resident cardiac cells. To investigate possible auto /paracrine effects, cardiomyocytes and cardiac fibroblasts were individually treated with recombinant murine MIF, which in turn induced Mif expression and the expression of pro-inflammatory genes in cardiac fibroblasts. Cardiomyocytes did not respond to recombinant MIF with pro-inflammatory gene expression. While MIF stimulation alone did not change the expression of pro-fibrotic genes in cardiac fibroblasts, ischemia reduced their expression. Mimicking the increased MIF levels during MI, we exposed cardiac fibroblasts to simulated ischemia in the presence of MIF, which led to further reduced expression of pro-fibrotic genes. The presented data show that MIF was expressed by resident cardiac cells during MI. In vitro, Mif expression was induced by different external stimuli in cardiomyocytes and cardiac fibroblasts. Addition of recombinant MIF protein increased the expression of pro-inflammatory genes in cardiac fibroblasts including Mif expression itself. Thereby, cardiac fibroblasts may amplify Mif expression during ischemia promoting cardiomyocyte survival.
Rat atrial engineered heart tissue: a new in vitro model to study atrial biology
Basic Research in Cardiology, September 2018
Krause J, Löser A, Lemoine MD, Christ T, Scherschel K, Meyer C, Blankenberg S, Zeller T, Eschenhagen T, Stenzig J
Abstract: Engineered heart tissue (EHT) from rat cells is a useful tool to study ventricular biology and cardiac drug safety. Since atrial and ventricular cells differ significantly, EHT and other 3D cell culture formats generated from ventricular cells have been of limited value to study atrial biology. To date, reliable in vitro models that reflect atrial physiology are lacking. Therefore, we established a novel EHT model using rat atrial cells (atrial EHT, aEHT) to assess atrial physiology, contractility and drug response. The tissue constructs were characterized with regard to gene expression, histology, electrophysiology, and the response to atrial-specific drugs. We observed typical functional properties of atrial tissue in our model such as more regular spontaneous beating with lower force, shorter action potential duration, and faster contraction and relaxation compared to ventricular EHT (vEHT). The expression of atrial-specific genes and proteins was high, whereas ventricle-specific transcripts were virtually absent. The atrial-selective drug carbachol had a strong negative inotropic and chronotropic effect on aEHT only. Taken together, the results demonstrate the feasibility of aEHT as a novel atrial 3D model and as a benchmark for tissue engineering with human induced pluripotent stem cell-derived atrial-like cardiomyocytes. Atrial EHT faithfully recapitulates atrial physiology and shall be useful to study atrial molecular physiology in health and disease as well as drug response.
Precursor proadrenomedullin influences cardiomyocyte survival and local inflammation related to myocardial infarction
Proceedings of the National Academy of Sciences of the United States of America, September 2018
Hinrichs S, Scherschel K, Krüger S, Neumann JT, Schwarzl M, Yan I, Warnke S, Ojeda FM, Zeller T, Karakas M, Keller T, Meyer C, Blankenberg S, Westermann D, Lindner D
Abstract: Increased adrenomedullin (ADM) levels are associated with various cardiac diseases such as myocardial infarction (MI). ADM is cleaved off from the full-length precursor protein proadrenomedullin (ProADM) during its posttranslational processing. To date, no biological effect of ProADM is reported, while ADM infusion leads to antiapoptotic effects and improved cardiac function. Using an MI mouse model, we found an induction of ProADM gene as well as protein expression during the early phase of MI. This was accompanied by apoptosis and increasing inflammation, which substantially influence the post-MI remodeling processes. Simulating ischemia in vitro, we demonstrate that ProADM expression was increased in cardiomyocytes and cardiac fibroblasts. Subsequently, we treated ischemic cardiomyocytes with either ProADM or ADM and found that both proteins increased survival. This effect was diminishable by blocking the ADM1 receptor. To investigate whether ProADM and ADM play a role in the regulation of cardiac inflammation, we analyzed chemokine expression after treatment of cells with both proteins. While ProADM induced an expression of proinflammatory cytokines, thus promoting inflammation, ADM reduced chemokine expression. On leukocytes, both proteins repressed chemokine expression, revealing antiinflammatory effects. However, ProADM but not ADM dampened concurrent activation of leukocytes. Our data show that the full-length precursor ProADM is biologically active by reducing apoptosis to a similar extent as ADM. We further assume that ProADM induces local inflammation in affected cardiac tissue but attenuates exaggerated inflammation, whereas ADM has low impact. Our data suggest that both proteins are beneficial during MI by influencing apoptosis and inflammation.
Impact of Intracardiac Neurons on Cardiac Electrophysiology and Arrhythmogenesis in an Ex Vivo Langendorff System
Journal of Visualized Experiments, May 2018
Jungen C, Scherschel K, Bork NI, Kuklik P, Eickholt C, Kniep H, Klatt N, Willems S, Nikolaev VO, Meyer C
Abstract: Since its invention in the late 19th century, the Langendorff ex vivo heart perfusion system continues to be a relevant tool for studying a broad spectrum of physiological, biochemical, morphological, and pharmacological parameters in centrally denervated hearts. Here, we describe a setup for the modulation of the intracardiac autonomic nervous system and the assessment of its influence on basic electrophysiology, arrhythmogenesis, and cyclic adenosine monophosphate (cAMP) dynamics. The intracardiac autonomic nervous system is modulated by the mechanical dissection of atrial fat pads-in which murine ganglia are located mainly-or by the usage of global as well as targeted pharmacological interventions. An octapolar electrophysiological catheter is introduced into the right atrium and the right ventricle, and epicardial-placed multi-electrode arrays (MEA) for high-resolution mapping are used to determine cardiac electrophysiology and arrhythmogenesis. Förster resonance energy transfer (FRET) imaging is performed for the real-time monitoring of cAMP levels in different cardiac regions. Neuromorphology is studied by means of antibody-based staining of whole hearts using neuronal markers to guide the identification and modulation of specific targets of the intracardiac autonomic nervous system in the performed studies. The ex vivo Langendorff setup allows for a high number of reproducible experiments in a short time. Nevertheless, the partly open nature of the setup (e.g., during MEA measurements) makes constant temperature control difficult and should be kept to a minimum. This described method makes it possible to analyze and modulate the intracardiac autonomic nervous system in decentralized hearts.
Ventricular tachycardia in ischemic heart disease: the sympathetic heart and its scars
American Journal of Physiology - Heart and Circulatory Physiology, March 2017
Meyer C, Scherschel K
Disruption of cardiac cholinergic neurons enhances susceptibility to ventricular arrhythmias
Nature Communications, January 2017
Jungen C, Scherschel K, Eickholt C, Kuklik P, Klatt N, Bork N, Salzbrunn T, Alken F, Angendohr S, Klene C, Mester J, Klöcker N, Veldkamp MW, Schumacher U, Willems S, Nikolaev VO, Meyer C
Abstract: The parasympathetic nervous system plays an important role in the pathophysiology of atrial fibrillation. Catheter ablation, a minimally invasive procedure deactivating abnormal firing cardiac tissue, is increasingly becoming the therapy of choice for atrial fibrillation. This is inevitably associated with the obliteration of cardiac cholinergic neurons. However, the impact on ventricular electrophysiology is unclear. Here we show that cardiac cholinergic neurons modulate ventricular electrophysiology. Mechanical disruption or pharmacological blockade of parasympathetic innervation shortens ventricular refractory periods, increases the incidence of ventricular arrhythmia and decreases ventricular cAMP levels in murine hearts. Immunohistochemistry confirmed ventricular cholinergic innervation, revealing parasympathetic fibres running from the atria to the ventricles parallel to sympathetic fibres. In humans, catheter ablation of atrial fibrillation, which is accompanied by accidental parasympathetic and concomitant sympathetic denervation, raises the burden of premature ventricular complexes. In summary, our results demonstrate an influence of cardiac cholinergic neurons on the regulation of ventricular function and arrhythmogenesis.
Development of nonfibrotic left ventricular hypertrophy in an ANG II-induced chronic ovine hypertension model
Physiological Reports, September 2016
Klatt N*, Scherschel K*, Schad C, Lau D, Reitmeier A, Kuklik P, Muellerleile K, Yamamura J, Zeller T, Steven D, Baldus S, Schäffer B, Jungen C, Eickholt C, Wassilew K, Schwedhelm E, Willems S, Meyer C
Abstract: Hypertension is a major risk factor for many cardiovascular diseases and leads to subsequent concomitant pathologies such as left ventricular hypertrophy (LVH). Translational approaches using large animals get more important as they allow the use of standard clinical procedures in an experimental setting. Therefore, the aim of this study was to establish a minimally invasive ovine hypertension model using chronic angiotensin II (ANG II) treatment and to characterize its effects on cardiac remodeling after 8 weeks. Sheep were implanted with osmotic minipumps filled with either vehicle control (n = 7) or ANG II (n = 9) for 8 weeks. Mean arterial blood pressure in the ANG II-treated group increased from 87.4 ± 5.3 to 111.8 ± 6.9 mmHg (P = 0.00013). Cardiovascular magnetic resonance imaging showed an increase in left ventricular mass from 112 ± 12.6 g to 131 ± 18.7 g after 7 weeks (P = 0.0017). This was confirmed by postmortem measurement of left ventricular wall thickness which was higher in ANG II-treated animals compared to the control group (18 ± 4 mm vs. 13 ± 2 mm, respectively, P = 0.002). However, ANG II-treated sheep did not reveal any signs of fibrosis or inflammatory infiltrates as defined by picrosirius red and H&E staining on myocardial full thickness paraffin sections of both atria and ventricles. Measurements of plasma high-sensitivity C-reactive protein and urinary 8-iso-prostaglandin F2α were inconspicuous in all animals. Furthermore, multielectrode surface mapping of the heart did not show any differences in epicardial conduction velocity and heterogeneity. These data demonstrate that chronic ANG II treatment using osmotic minipumps presents a reliable, minimally invasive approach to establish hypertension and nonfibrotic LVH in sheep.
Accumulation of free oligosaccharides and tissue damage in cytosolic α-mannosidase (Man2c1)-deficient mice
Journal of Biological Chemistry, April 2014
Paciotti S, Persichetti E, Klein K, Tasegian A, Duvet S, Hartmann D, Gieselmann V, Beccari T
Abstract: Free Man(7-9)GlcNAc2 is released during the biosynthesis pathway of N-linked glycans or from misfolded glycoproteins during the endoplasmic reticulum-associated degradation process and are reduced to Man5GlcNAc in the cytosol. In this form, free oligosaccharides can be transferred into the lysosomes to be degraded completely. α-Mannosidase (MAN2C1) is the enzyme responsible for the partial demannosylation occurring in the cytosol. It has been demonstrated that the inhibition of MAN2C1 expression induces accumulation of Man(8-9)GlcNAc oligosaccharides and apoptosis in vitro. We investigated the consequences caused by the lack of cytosolic α-mannosidase activity in vivo by the generation of Man2c1-deficient mice. Increased amounts of Man(8-9)GlcNAc oligosaccharides were recognized in all analyzed KO tissues. Histological analysis of the CNS revealed neuronal and glial degeneration with formation of multiple vacuoles in deep neocortical layers and major telencephalic white matter tracts. Enterocytes of the small intestine accumulate mannose-containing saccharides and glycogen particles in their apical cytoplasm as well as large clear vacuoles in retronuclear position. Liver tissue is characterized by groups of hepatocytes with increased content of mannosyl compounds and glycogen, some of them undergoing degeneration by hydropic swelling. In addition, lectin screening showed the presence of mannose-containing saccharides in the epithelium of proximal kidney tubules, whereas scattered glomeruli appeared collapsed or featured signs of fibrosis along Bowman’s capsule. Except for a moderate enrichment of mannosyl compounds and glycogen, heterozygous mice were normal, arguing against possible toxic effects of truncated Man2c1. These findings confirm the key role played by Man2c1 in the catabolism of free oligosaccharides.
Hepatoma-derived growth factor and nucleolin exist in the same ribonucleoprotein complex
BMC Biochemistry, January 2013
Bremer S, Klein K, Sedlmaier A, Abouzied M, Gieselmann V, Franken S
Abstract: BACKGROUND: Hepatoma-derived growth factor (HDGF) is a protein which is highly expressed in a variety of tumours. HDGF has mitogenic, angiogenic, neurotrophic and antiapoptotic activity but the molecular mechanisms by which it exerts these activities are largely unknown nor has its biological function in tumours been elucidated. Mass spectrometry was performed to analyse the HDGFStrep-tag interactome. By Pull-down-experiments using different protein and nucleic acid constructs the interaction of HDGF and nucleolin was investigated further. RESULTS: A number of HDGFStrep-tag copurifying proteins were identified which interact with RNA or are involved in the cellular DNA repair machinery. The most abundant protein, however, copurifying with HDGF in this approach was nucleolin. Therefore we focus on the characterization of the interaction of HDGF and nucleolin in this study. We show that expression of a cytosolic variant of HDGF causes a redistribution of nucleolin into the cytoplasm. Furthermore, formation of HDGF/nucleolin complexes depends on bcl-2 mRNA. Overexpression of full length bcl-2 mRNA increases the number of HDGF/nucleolin complexes whereas expression of only the bcl-2 coding sequence abolishes interaction completely. Further examination reveals that the coding sequence of bcl-2 mRNA together with either the 5' or 3' UTR is sufficient for formation of HDGF/nucleolin complexes. When bcl-2 coding sequence within the full length cDNA is replaced by a sequence coding for secretory alkaline phosphatase complex formation is not enhanced. CONCLUSION:The results provide evidence for the existence of HDGF and nucleolin containing nucleoprotein complexes which formation depends on the presence of specific mRNAs. The nature of these RNAs and other components of the complexes should be investigated in future.
Lysosomal di-N-acetylchitobiase-deficient mouse tissues accumulate Man2GlcNAc2 and Man3GlcNAc2
Biochimica et Biophysica Acta, July 2012
Persichetti E, Klein K, Paciotti S, Lecointe K, Balducci C, Franken S, Duvet S, Matzner U, Roberti R, Hartmann D, Gieselmann V, Beccari T
Abstract: Most lysosomal storage diseases are caused by defects in genes encoding for acidic hydrolases. Deficiency of an enzyme involved in the catabolic pathway of N-linked glycans leads to the accumulation of the respective substrate and consequently to the onset of a specific storage disorder. Di-N-acetylchitobiase and core specific α1-6mannosidase represent the only exception. In fact, to date no lysosomal disease has been correlated to the deficiency of these enzymes. We generated di-N-acetylchitobiase-deficient mice by gene targeting of the Ctbs gene in murine embryonic stem cells. Accumulation of Man2GlcNAc2 and Man3GlcNAc2 was evaluated in all analyzed tissues and the tetrasaccharide was detected in urines. Multilamellar inclusion bodies reminiscent of polar lipids were present in epithelia of a scattered subset of proximal tubules in the kidney. Less constantly, enlarged Kupffer cells were observed in liver, filled with phagocytic material resembling partly digested red blood cells. These findings confirm an important role for lysosomal di-N-acetylchitobiase in glycans degradation and suggest that its deficiency could be the cause of a not yet described lysosomal storage disease.
* These authors contributed equally.
Media
-
A Common Atrial Fibrillation Procedure Is Aided by Damaging Neurons , The Scientist (2019)
-
Neue Erfolgskontrolle für Katheterbehandlung bei Vorhofflimmern , DZHK (2019)
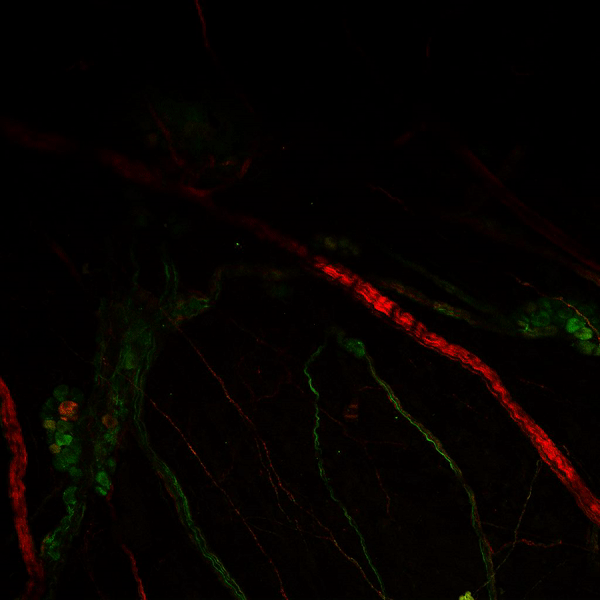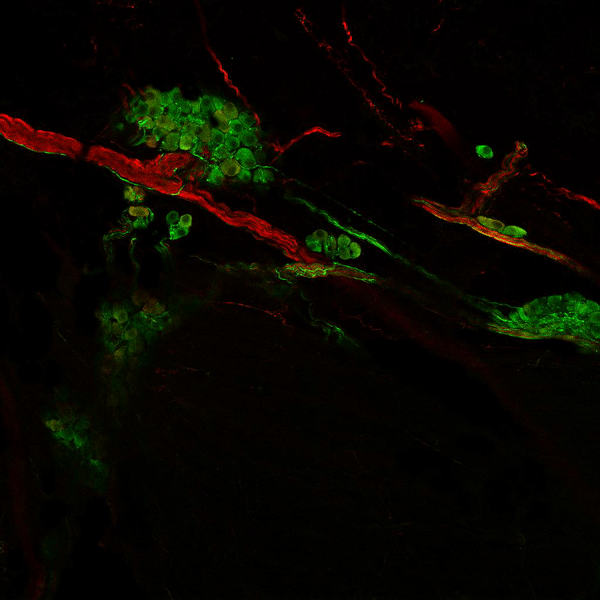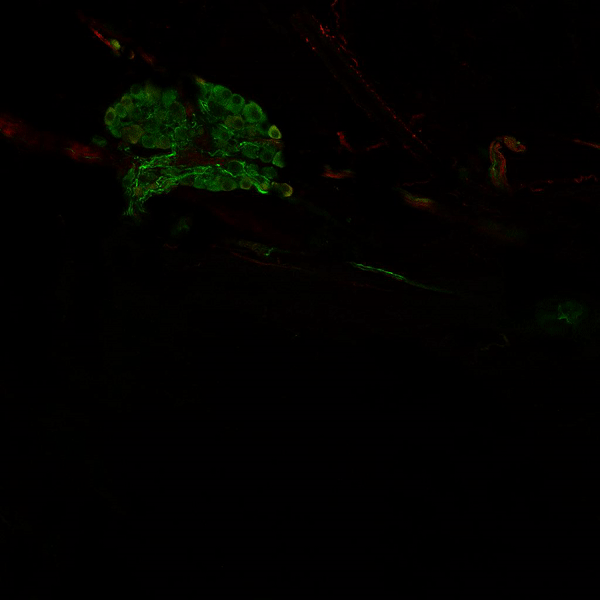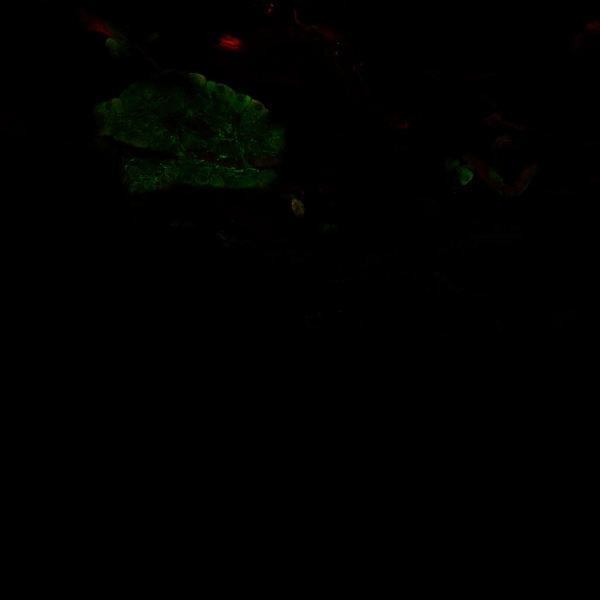
Visualisation of the “Brain within the Heart”
This animation shows the nervous system of a mouse heart – which also exists in the human heart. Stained in green are parasympathetic neurons and nerves, stained in red are sympathetic neurons and nerves.
Together, these two branches of the autonomic nervous system modulate our heart rhythm beat-to-beat. This remarkable network allows our body to adapt to environmental changes – be it an immediate danger (fight or flight) – or relaxation (rest and digest).